ELISA and rapid tests
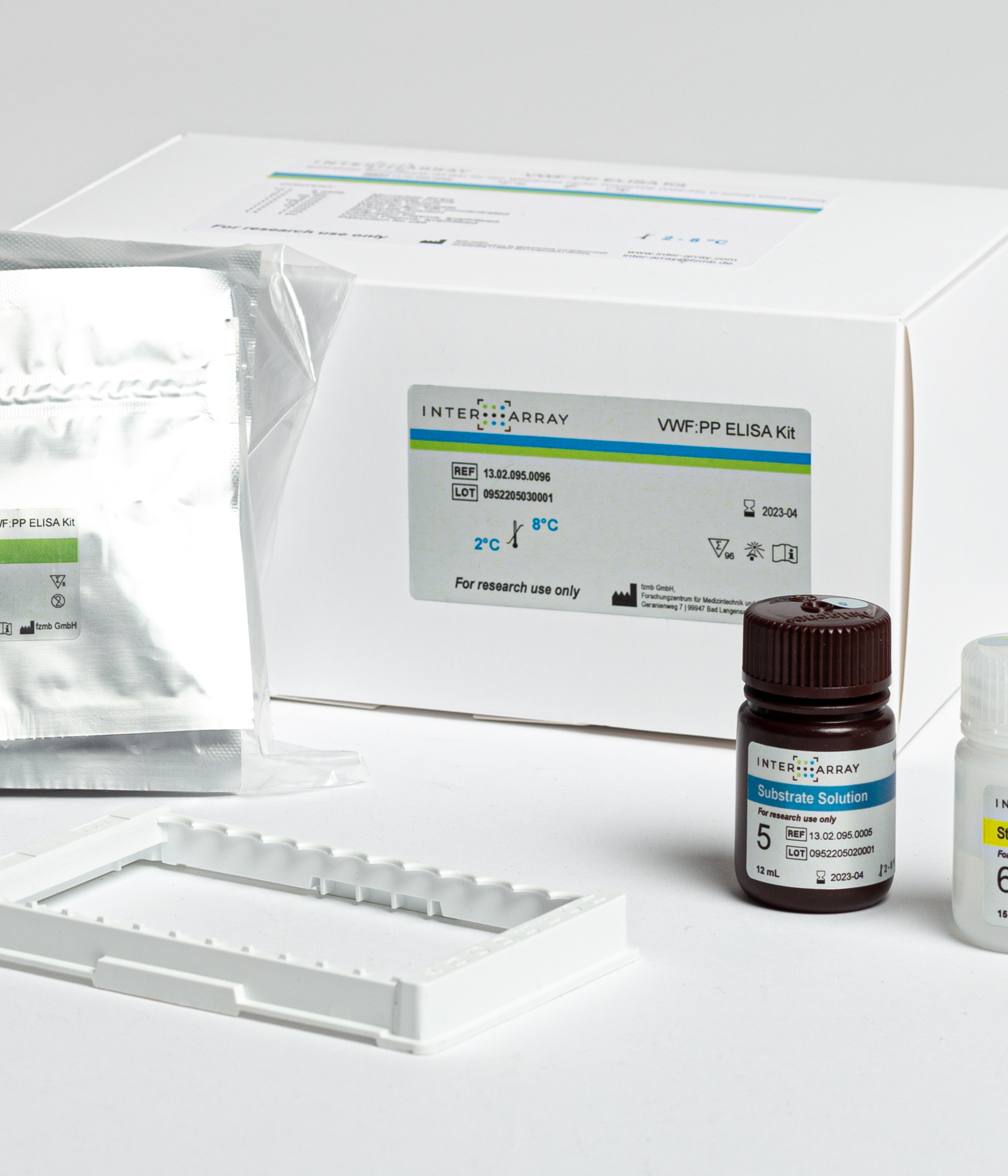
vWF-PP ELISA
ELISA for quant. measurement of the von Willebrand factor propeptide (vWF-PP)
Von Willebrand factor (VWF) has several important functions in primary hemostasis. The multimeric protein is found in plasma, platelets and endothelial cells. VWF is also a carrier protein and stabilizer for clotting factor VIII (FVIII) in plasma.
During the biosynthesis of VWF multimers, a 100 kDa glycoprotein propeptide (VWF:PP) is proteolytically cleaved and released into plasma. In certain types of VWF disorders, including inherited or acquired forms, mutations, and some other diseases, abnormal levels of both VWF:PP and VWF:AG (VWF antigen) or VWF activity may be detected, as reflected by the ratio of VWF:PP and VWF:AG.
People with VWF disease show an increased tendency to bleed, e.g. epistaxis, bleeding gums, hematomas or excessive bleeding after dental or other surgical procedures. In extreme cases of absolute deficiency (VWF disease type 3), life-threatening bleeding can occur. In blood, VWF and VWF:PP have very different half-lives (12 versus 2 hours). Measurement of VWF:PP, in addition to VWF:AG, is an important tool for characterizing the nature of VWF deficiency, particularly in individuals with a shortened plasma half-life of VWF.
The wells of the microplate strips included in this kit are coated with a monoclonal antibody directed against VWF:PP. The sample is pipetted into a well followed by a second monoclonal antibody against VWF:PP conjugated to an enzyme. VWF:PP binds to the antibody bound to the solid phase and is immobilized.
The second antibody with the conjugated enzyme also binds to the immobilized VWF:PP. After incubation and washing steps, all unbound material is removed, and the added substrate is cleaved by the bound enzyme of the conjugate, releasing a dye in proportion to the bound VWF:PP. The reaction is stopped after a certain time and the absorption is measured. This shows the concentration of VWF:PP.
The assay is calibrated by parallel measurement of the supplied calibrator and its dilutions against a calibration curve. Quality control is possible by simultaneous analysis of the control plasma included in the kit.
Product number: 13.02.095.0096 (processing by pipetting robot), 13.02.095.1096 (manual processing)
Product Name: INTER-ARRAY VFW:PP ELISA Kit – Enzyme linked immunosorbent assay (ELISA) for the quantitative determination of von Willebrand factor propeptide (VWF:PP) in human blood plasma. For research purposes only.
Product content: The ready-to-use kit is available in two versions from our distributors: for manual processing in the laboratory or for use with automated pipetting systems. In addition to the individually packaged antibody-coated microplate strips, all reagents required for processing as well as lyophilized calibrator and control plasma are included.
Literature/ Data sheets
Inquiries and orders
Bot. Tox. A Abicap SL 24 Kit / Bot. Tox. B Abicap SL 24 Kit
Immunoassay for the detection of botulinum toxin A or botulinum toxin B
The Abicap columns used contain three 3-dimensional filters: two spacer filters (1.6 x 5 mm) coated with BSA and, in between, a measurement filter (2.5 x 5 mm) coated with capture antibodies directed against botulinum toxin A1/2 and botulinum toxin B, respectively.
In the first step, the pre-incubated sample is applied to the Abicap column and captured by the specific antibody on the filter surface. The sandwich complexes are detected by addition of streptavidin-poly-horseradish peroxidase and tetramethyl-benzidine substrate solution. The enzymatic reaction results in a blue precipitate on the filter surface with an optical density proportional to the toxin concentration.
Measuring range in sample dilution buffer: 5-100 U/ml or 5-500 U/ml.
Product number: 13.02.071.0240 (Bot. Tox. A Abicap SL 24 Kit) and 13.02.072.0240 (Bot. Tox. B Abicap SL 24 Kit)
Product name: Bot. Tox. A Abicap SL 24 Kit and Bot. Tox. B Abicap SL 24 Kit – Test kits for the determination of botulinus toxin A or botulinus toxin B. For research purposes only.
Product content: In addition to the individually packaged Abicap columns loaded with antibody-coated microfilters, all reagents required for processing are included.
Inquiries and orders
MMP9 rapid test
Lateral Flow Assay for the detection of MMP-9 (matrix metalloprotease 9) in body fluids of horses
Usage
The MMP-9 cassette test is a rapid visual test for the qualitative detection of MMP-9 in equine body fluid. This kit is intended as an aid in the assessment of protease activity and is intended for in vitro diagnostic use by professionals only.
Background information
Matrix metalloproteinases (MMPs) play a key role in degenerative processes, making them a predestined biomarker in equine inflammatory diseases (Clutterbuck et al. 2010). MMP-9 is causally involved in cartilage degradation in joint disease (Clegg and Carter 1999; Clegg et al. 1997) and is therefore used as a marker in lameness. MMP-9 concentrations in plasma and peritoneal fluid are also elevated in horses with colic symptoms and a positive sepsis score (Barton et al. 2021).
During surgical treatment of recurrent uveitis (ERU) by vitrectomy, MMP-9 rapid analysis can be used for intraoperative monitoring to assess the progress of surgery.
The equine-specific MMP-9 cassette test bypasses time-consuming and labor-intensive laboratory methods such as zymography and ELISA and provides a result in minutes. However, for accurate quantification of MMP-9 content, the equine-specific MMP-9 ELISA should be performed.
Test principle
The MMP-9 test is a sandwich immunoassay for the detection of MMP-9 in equine body fluid by visual interpretation of color development in the test cassette. The membrane was coated with an antibody against equine MMP-9 in the test line region (T). During the assay, the diluted sample reacts with a stained conjugate (anti-equine-MMP-9 antibody gold conjugate) that has been added to the pad inside the test cassette.
The mixture moves chromatographically across the membrane by capillary action. If MMP-9 is present in the sample, a colored line with a specific antibody-antigen conjugate complex forms in the test line region (T) of the membrane. This complex consists of a stained anti-MMP-9 antibody, MMP-9 from the sample, and the antibody fixed on the membrane in the test line region (T).
On the other hand, a colored line always appears in the control region (C). For this purpose, another antigen-antibody reaction (with antimouse antibodies) is used. This control line serves as a procedural indicator of the proper functioning of the test. It indicates that the test procedure has been performed correctly and that the sample has flowed properly across the membrane.
A pronounced color development in the test line region (T) indicates a positive result (MMP-9 present in the sample). The absence of a color line in the test line region (T) indicates a negative result (no MMP-9 present in the sample).
MMP9 ELISA
ELISA for the detection of MMP9 (equine matrix metalloprotease 9)
in body fluids of horses
Product name: eqMMP-9 FAST ELISA Kit
The MMP-9 ELISA is an immunological enzymatic detection method for the detection of equine matrix metalloproteinase 9 (eqMMP-9, gelatinase B, 92 kDa gelatinase, 92 kDa type IV collagenase, MMP-9) in equine body fluids.
eqMMP can be detected in various body fluids of horses including synovial fluid, vitreous fluid and bronchoalveolar lavage fluid (BALF).
The monoclonal antibodies used in the kit were generated against native equine MMP-9.
The ELISA is suitable for use by qualified personnel in the veterinary diagnostic field.
Inquiries and orders
About MMP-9
MMP-9, also called 92 kDa type IV collagenase, 92 kDa gelatinase or gelatinase B, is involved in many physiological processes and on the other hand in the pathophysiological degradation of the extracellular matrix for example in articular cartilage in joint diseases, in the vitreous body of the eye in equine recurrent uveitis (ERU), in peritoneal fluid in endotoxemia of equine colic, in bronchoalveolar lavage fluid (BALF) in recurrent airway obstruction (RAO, COPD), in wound exudate in wound healing disorders, in tumors and other diseases. It is thus a predestined biomarker in equine inflammatory diseases.
Literature
Clegg, P.D. and Carter, S.D. (1999) Matrix metalloproteinase-2 and -9 are activated in joint diseases. Equine Vet J 31, 324-330.
Clegg, P.D., Coughlan, A.R., Riggs, C.M. and Carter, S.D. (1997) Matrix metalloproteinases 2 and 9 in equine synovial fluids. Equine Vet J 29, 343-348.
Barnewitz, D., Karakine E., Richter I.-G., Lerchbacher J. (2015) Importance of MMP-9 in lameness diagnostics. Der praktische Tierarzt 96th year, pp. 1124 ff.
Clutterbuck, A.L., Harris, P., Allaway, D. and Mobasheri, A. (2010) Matrix metalloproteinases in inflammatory pathologies of the horse. Vet J 183, 27-38.
Barton, A.K., et al. (2021). MMP-9 Concentration in Peritoneal Fluid Is a Valuable Biomarker Associated with Endotoxemia in Equine Colic. Mediators of Inflammation Vol. 2021.
fTLI ELISA
ELISA for the quantitative detection of felin trypsinogen in serum
The most important laboratory parameter for pancreatic insufficiency in cats is feline trypsin-like immunoreactivity (fTLI). Here, both trypsin and its precursor, trypsinogen, are determined in the serum.

Product decription: fTLI-ELISA
Product content: fTLI kit 10x each
-
- 96 well microtiter plate with anti-fTLI coated capture antibody
(individually removable cavities or strips)
-
- Dilution buffer
- Wash buffer
- Substrate solution
- Stop solution
- Antibody conjugate (ready to use)
- Trypsinogen Standard S1 (ready to use)
- Control serum (fTLI 10 – 14 μg/L)
Price: Upon request
Product description
The determination of feline trypsin-like immunoreactivity (fTLI) concentration in blood serum is a specific parameter for exocrine pancreatic function in laboratory diagnostics.
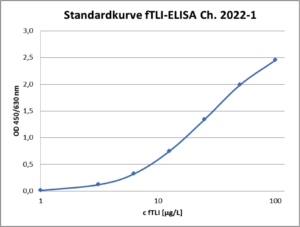
The fTLI Screening Test is a highly specific ELISA in microplate format for the quantitative detection of felin trypsinogen from serum samples. The determination is performed directly from serum samples of cats, without prior isolation in a measuring range from 3 to 100 µg/L. The test result can be read within 90 min after sample application. The special ELISA setup enables simple, well-accurate and rapid sample analysis with little handling effort.
Inquiries and orders
Rapid test for the detection of LukF-P83 in S.aureus
Bacterial infections of the udder
Mastitis is an inflammatory disease of the udder caused, among other things, by the infectious agent Staphylococcus aureus is caused. Early detection and treatment of the infection is crucial to prevent it spreading in the dairy herd.
Infection detection via rapid test
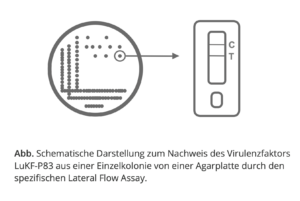
The strains of S. aureus relevant for mastitis in Europe can be identified via the gene product lukF-P83 (coded for the exotoxin leukocidin). We have therefore developed an easy-to-perform lateral flow assay. This test is specific for the bacterial exotoxin (LukF-P83) of S. aureus.
Secure special offer now
We support your work with the production of a series of 500 LukF- P83 Lateral Flow Assay (20 x 25 antigen rapid test) at the one-time promotional price of EUR 3,180.00 instead of EUR 4,890.00 plus VAT. VAT and shipping.
This offer is valid until 30.09.2024.
Contact
Literature
Schlotter K., Ehricht R., Hotzel H.; Monecke S., Pfeffer M., Donat K. 2012. leukocidin genes lukF-P83 and lukM are associated with Staphylococcus aureus clonal complexes 151, 479 and 133 isolated from bovine udder infections in Thuringia, Germany. Veterinary research 43. https://doi.org/10.1186/1297-9716-43-42
Antibodies and antigens
Overview antibodies
At the fzmb, we use hybridoma technology to develop specific monoclonal antibodies in the mouse host organism. In addition to antibody production, we can also supply the equivalent antigen (recombinant and native proteins, peptides, microorganisms and their structures).
For rapid downstream development, we offer antibody characterization and matched pair analysis. The antibodies can therefore be used in high-quality and effective test systems.
In addition to monoclonal antibodies (mouse, purification via affinity chromatography), we also offer the corresponding hybridoma cell lines. Our production conforms to ISO 9001 and antibody production under ISO 13485 is possible on request.
Monoclonal polyhisitidine antibodies (6xHis-tag)
Polyhistidine tags are used, for example, to purify recombinant proteins by affinity chromatography. The anti-polyhistidine tag antibodies are suitable, for example, for the detection of the His tag on these recombinant proteins.
We offer various cell lines and antibodies for anti-polyhistidine tag in our mylab system solutions/laboratory and POCT diagnostics business unit.
Monoclonal Legionella antibodies
Legionella are gram-negative, aerobic bacteria. According to the RKI, over 60 species with almost 80 different serogroups are known.
We offer various cell lines and antibodies against different servovars in our mylab system solutions/laboratory and POCT diagnostics business unit.
Monoclonal AK against methicillin-resistant S. aureus
Methicillin-resistant S. aureus strains (MRSA) are characterized by the fact that they are resistant not only to all β-lactam antibiotics currently available on the market, but also to the penicillase-stable antibiotics methicillin and oxacillin. The cause of this resistance lies in the mecA gene, which encodes a modified penicillin-binding protein (PBP2a).
Panton-Valentine leukocidin (PVL) is a strong virulence factor of Staphylococcus aureus. This exotoxin leads to the lysis of leukocytes by forming pores in the cytoplasmic membrane. PVL-producing S. aureus pathogens are highly pathogenic and cause severe infections of the skin and soft tissue, including lethal pneumonia.
We offer monoclonal antibodies (incl. matched pairs) and AK-producing hybridoma cell lines against penicillin-binding protein 2a (PBP2a) and against Panton-Valentine leukocidin (PVL) in our mylab system solutions/laboratory and POCT diagnostics business unit.
Monoclonal antibodies against paratuberculosis
Paratuberculosis, also known as “Johne’s disease”, is a disease characterized by Mycobacterium avium ssp. paratuberculosis (MAP), a chronic inflammatory bowel disease that occurs predominantly in ruminants. We have developed a set of antibodies for the immunological detection of paratuberculosis.
We offer various monoclonal antibodies and AK-producing hybridoma cell lines against Mycobacterium avium ssp. paratuberculosis (MAP) in our mylab system solutions/laboratory and POCT diagnostics division.
Monoclonal antibodies against mycotoxins
Mycotoxins, also known as “mold toxins”, are metabolic products formed by fungi that can be contained in agricultural products and food. Mycotoxins can have various toxic effects on vertebrates, even in very small quantities. A disease caused by mycotoxins is known as mycotoxicosis. The most common mycotoxins are deoxynivalenol (DON) and zearalenone (ZEA), which are produced by the genus Fusarium.
We offer various monoclonal antibodies and AK-producing hybridoma cell lines against the mycotoxins deoxynivalenol (DON) and zearalenone (ZEA) produced by the genus Fusarium through our mylab system solutions/laboratory and POCT diagnostics business unit.
Overview of antibody-producing cell lines
We offer a selection of antibody-producing hybridoma cell lines (mouse). We look forward to your interest in commercial use. Please contact us for the price of a desired cell line.
- Alpha1 proteinase inhibitor (dog) against cannie alpha1PI
- Archaea ssp. (e.g. Methanosarcinae) against M. mazei Gs14, M.mazei DSM2053, M. flavesc.
- BBI against Bowman-Birk Inhbitor
- Clostridium botulinum neurotoxin C against BoNT-C
- Coxiella burnetii against Coxiella burnetii Phase I or II
- DON against deoxynivalenol
- Fluorescent dyes and quenchers against FITC, Dyeomics 405, Dyomics 495, Dyeomics Quencher Q1, Dyeomics Quencher Q3
- Factor X against human factor X
- Factor Xa against human faxctor Xa
- American foulbrood (AFB)
- feline trypsinogen g
- Heparin Binding Protein against human heparin
- HIS-Tag against poly6-HIS-Tag
- Legionella pneumophila against various serogroups from SG1 to SG14
- Lunasin
- MAP against Mycobacterium avium paratuberculosis (inactivated cells)
- eMMP9 against equine matrix metallo proteinase 9)
- NDV against Newcastle Disease Virus
- fNTproBNP (feline natriuretic brain peptide)
- cNTproBNP (canine Natriuretic Brain Peptide)
- PMSG against equine pregnant mare serum gonadotropin
- S. aureus LukF-P83 against Panton valentin leukocidin LukF-P83
- S. aureus PVL against Panton valentin leukocidin LukF-PV
- Salmonella ssp. against Salmonella enterititis
Contact
PBP2a antibody for the detection of MRSA
Meticillin-resistant S. aureus (MRSA)
The Gram-positive bacterium Staphylococcus aureus is found on the skin and mucous membranes of one in four people. S. aureus is generally harmless, but can cause serious illnesses such as pneumonia, bone or heart valve infections, especially in weakened people.
Infections with this Gram-positive bacterium are treated with antibiotics from the group of so-called beta-lactams. The β-lactam antibiotics, which include penicillins, chephalosporins and carpabenems (external link: overview of antibiotics), prevent the cell wall of Gram-positive bacteria from being rebuilt so that they are no longer viable.
A growing problem is the increase in S. aureus strains that are resistant to β-lactam antibiotics. These strains are known as meticillin-resistant S. aureus (MRSA).
Methicillin-resistant S. aureus (MRSA) are characterized by the fact that they are resistant not only to all β-lactam antibiotics currently available on the market, but also to the penicillase-stable antibiotics methicillin and oxacillin.
Modified penicillin binding protein (PBP2a) leads to resistance
The cause of this resistance lies in the mecA gene, which encodes a modified penicillin binding protein (PBP2a, Penicillin Binding Protein-2a). This transpeptidase is involved in linking the bacterial cell wall components. However, β-lactam antibiotics can no longer bind to this modified penicillin binding protein and cell wall biosynthesis can proceed unhindered.
New antibodies against PBP2a
For selective detection of meticillin-resistant S. aureus (MRSA) strains, the fzmb has developed very potent PBP2a antibodies in cooperation with other research institutions and companies.
The PBP2a antibodies from our hybridoma cell lines can be used, for example, in immunological tests such as lateral flow assays (LFA) or ELISA. Corresponding matched pairs are available. The antibodies are produced in accordance with ISO 9001. Production according to ISO 13485 on request.
Product number: PBP-003a; PBP-004: PBP-006; PBP-007; PBP-012
Product name: Anti-PBP2a [MRSA] antibody, mouse monoclonal, affinity purified
Contact
Recombinant equine MMP-9
Product no. :
Product name: eqMMP-9 (recombinant)
Product description: Recombinant equine MMP-9 protein; Species: Horse; Suitable for: SDS-PAGE, WB, ELISA
Product content: lyophilized recombinant protein, 10µg
Price: upon request
Data sheets
Contact for information / order
Dr. Ina-Gabriele Richter
phone +49 (0)360 – 833 177
Native equine MMP-9
Product name: eqMMP-9 (native)
Product conent: 1 glass vial with 0.14 µg lyophilized enzyme (chromatography-purified)
Price: upon request
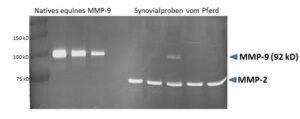
Product description
Native equine MMP-9 protein (active); species: horse; active: yes, suitable for: Functional studies (zymography, gelatinase assay), SDS-PAGE, WB, ELISA; chromatography-purified (Hibbs et al., 1985; Imai and Okada, 2008).
Literature
Hibbs, M.S., K.A. Hasty, J.M. Seyer, A.H. Kang, and C.L. Mainardi. 1985. Biochemical and immunological characterization of the secreted forms of human neutrophil gelatinase. J Biol Chem. 260:2493-2500.
Imai, K., and Y. Okada. 2008. Purification of matrix metalloproteinases by column chromatography. Nat Protoc. 3:1111-1124
Data sheets
Inquiries and order
Dr. Ina-Gabriele Richter
phone +49 (0)3603 – 833 177
Kits for genotyping
INTER-ARRAY Genotyping Kit CarbaResist
The company INTER-ARRAY is part of the fzmb. The INTER-ARRAY genotyping kit CarbaResist enables DNA-based detection of the most common carbapenemase genes of multidrug-resistant Gram-negative bacteria from bacterial cultures. In addition, the identification of some important Gram-negative bacteria (e.g. B. Pseudomonas aeruginosa, Klebsiella pneumoniae, Escherichia coli) are possible.
The probes for the detection of the target genes are immobilized on the ArrayWell. After isolation of RNA-free, unfragmented genomic DNA from pure and monoclonal colony material, the DNA is amplified using a linear PCR amplification protocol and only the antisense primer of the different targets and internally labeled with biotin.
The results are single-stranded DNA reaction products (ssDNA). In the next step, this biotin-labeled ssDNA will be transferred to the ArrayWell and hybridized to DNA oligonucleotide microarrays containing 230 probes for various carbapenemase and AmpC genes and other relevant antibiotic resistance genes.
After hybridization and subsequent washing, HRP-conjugated streptavidin binds to the hybridized biotin-labeled ssDNA strains and visualizes them in a subsequent enzymatic reaction. The same reaction takes place with the stain control.
The evaluation of the spots and their intensities is performed automatically on the basis of a digital image of the microarray with the INTER-VISION reader. The entire sample is automatically analyzed for the presence or absence of specific probes, matched against a database, and then outputs information on existing resistance and possible bacterial species.
Product number: 13.02.102.0960
Product name: INTER-ARRAY Genotyping Kit CarbaResist – Test kits for DNA-based identification of important multidrug-resistant Gram-negative bacteria and for characterization of important carbapenemases and other resistances. For research purposes only.
Product scope: The processing of the genotyping kit is divided into five sub-steps: Cell Lysis, DNA Labelling and Amplification, Hybridization, Detection and Staining. The kit contains reagents for performing 96 individual determinations. DNA of the E. coli strain Nord8 is included in the kit as reference material.
Inquiries and orders
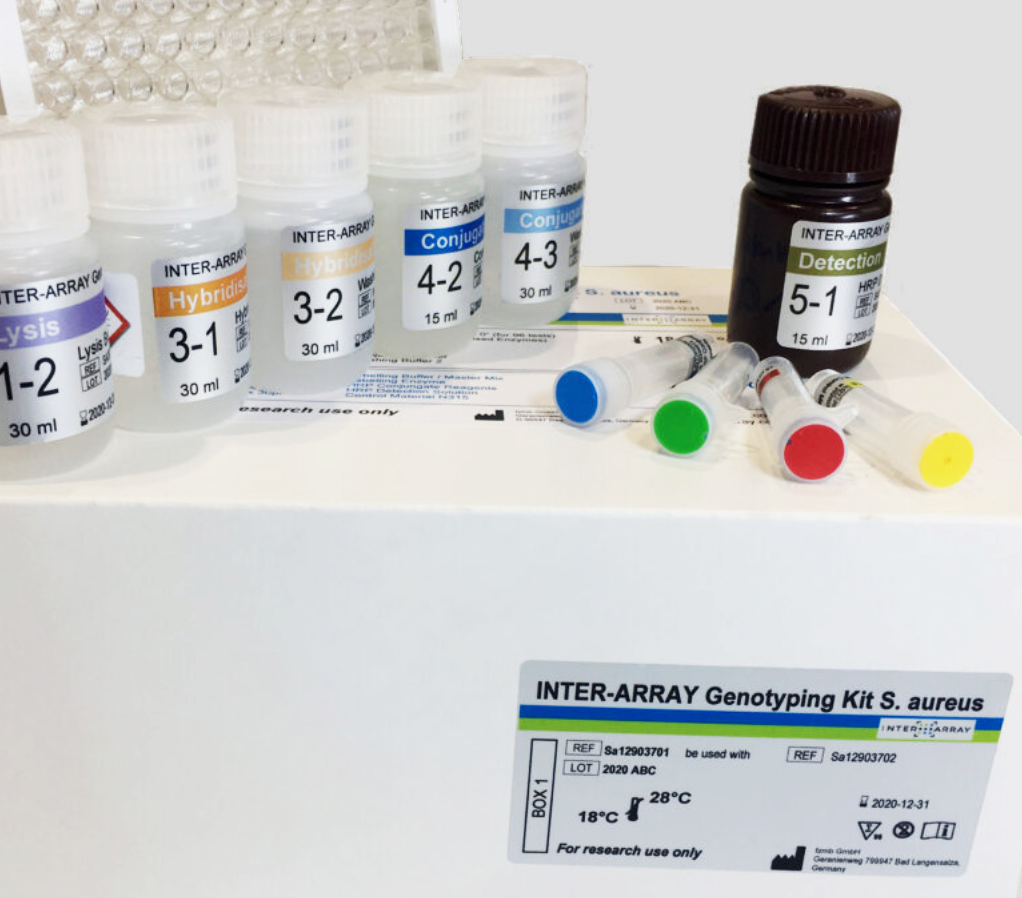
INTER-ARRAY Genotyping Kit S. aureus
INTER-ARRAY is part of the fzmb. The INTER-ARRAY Genotyping Kit S. aureus allows DNA-based detection of resistance genes and pathogenicity markers of Staphylococcus aureus and assignment of unknown S. aureus isolates to known strains. The probes for the detection of the target genes are immobilized on the ArrayWell.
After isolation of RNA-free, unfragmented genomic DNA from pure and monoclonal S. aureus colony material, the DNA is amplified using a linear PCR amplification protocol and only the antisense primer of the different targets and internally labeled with biotin. The results are single-stranded DNA reaction products (ssDNA).
Unlike standard PCR, which involves exponential amplification, linear amplification is less sensitive and therefore requires significantly more DNA starting material (0.5-2 μg). For this reason, the method is limited to colony material and cannot be performed on samples, e.g., from smears.
In the next step, this biotin-labeled ssDNA will be transferred to an ArrayWell and hybridized to DNA oligonucleotide microarrays containing 336 probes for various genetic markers of S. aureus strains. These genetic markers include a variety of species markers, virulence-associated genes for exotoxins, antibiotic resistance, MSCRAMMs, various enzymes, and other types of markers.
After hybridization and subsequent washing, HRP-conjugated streptavidin binds to the hybridized biotin-labeled ssDNA strains and visualizes them in a subsequent enzymatic reaction. The same reaction takes place with the stain control. The evaluation of the spots and their intensities is performed automatically on the basis of a digital image of the microarray with the INTER-VISION reader.
The overall pattern is automatically analyzed for the presence or absence of specific markers and compared to a database of strain profiles that allows assignment to clonal complexes and strains.
Product number: 13.02.101.0960
Product name: INTER-ARRAY Genotyping Kit S. aureus – Test kits for DNA-based determination of bacterial cultures of Staphylococcus aureus isolates. For research purposes only.
Product scope: The processing of the genotyping kit is divided into five sub-steps: Cell Lysis, DNA Labelling and Amplification, Hybridization, Detection and Staining. The kit contains reagents for performing 96 individual determinations. DNA of S. aureus strain N315 is included in the kit as reference material.
Inquiries and orders
On-site measurement: Food ingredients

mylab NIR Analyzer

Real-time monitoring for composition of meat and sausage products
The mylab NIR Analyzer MEAT is a highly precise, robust and easy-to-use measuring instrument. A special imaging technique (spectrometry) is used to analyze the composition of meat and sausage products.
The recorded NIR spectra and their automatic evaluation thus enable fast and cost-effective on-site analysis, e.g. in the production process. The non-contact and non-destructive measurement takes place within a few seconds and requires no additional chemicals.
The mylab NIR Analyzer MEAT is supplied with a comprehensive basic calibration for meat and sausage products. Adaptation to other products is possible at any time.
Strengths of the mylab NIR analyzer MEAT
- 21 parameters relevant in the meat industry can be measured in a few seconds
- small footprint and light weight, flexible to use
- easy to use 1-click software
- no chemical consumption
- Cost-effective
The mylab device can also be adapted to specific products.
Product no.: P006-001
Product name: mylab NIR- Analyzer MEAT
Product scope: 1 mylab NIR- Analyzer MEAT incl. Software and calibration models (L 34 cm x W 24 cm x H 32 cm; 5.5 kg), power supply (1x), power cord (1x), spare lamp (1x), Petri dish made of special glass (5x), spare dust filter (2x); operating instructions; total weight: 10 kg incl. Packing
Parameter
Determination of 21 parameters for meat and sausage products
- BIG 7 (according to EU-VO 1169/2011)
- pH value
- aW value
- BEFFE
- BEFFE in FE
- Extraneous water (via spring number) & others
The basic calibration covers the product groups fresh meat, raw sausage, scalded sausage and cooked sausage of beef and pork.
PDF Calibration Data Sheet Meat and Sausages (German)
PDF Technical Specifications (english)
Contact for further information
Alexander Mücke
+49(0)3603 – 833 193
Find more information at
mylab NIR Analyzer CF (custom-fit)
Real-time determination for food composition
The mylab NIR Analyzer CF is a high-precision instrument for the analysis of food ingredients. By means of recorded NIR spectra and their automatic evaluation, the system enables fast and cost-effective on-site analysis throughout the production process. The non-contact and non-destructive measurement takes place within a few seconds and requires no additional chemicals.
For the application, the mylab NIR Analyzer CF requires a specific calibration for the respective product (e.g. dairy products, ready meals, delicatessen salads, seeds, cereals, flour, feed, small animal feed). Based on our database (with calibrations) and supplemented by calibrations of your products, a high-precision measurement procedure is created.
Strengths of the mylab NIR Analyzer CF
- Data on relevant parameters measurable in a few seconds
- small footprint and light weight, flexible to use
- easy to use 1-click software
- no chemical consumption
- Cost-effective

Contact for further information
Alexander Mücke
+49(0)3603 – 833 193
Find more information at

